History and formulation of Polyurethane
Polyurethane
History and formulation
One calls urethane, or commonly "carbamate", any compound produced by the reaction of an isocyanate and an alcohol in accordance with the following reaction:
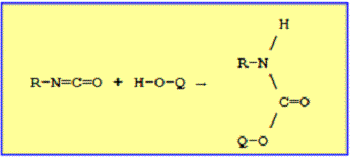
This reaction had been known for several decades when in 1937, Otto Bayer discovered how to make a plastic usable, free from polyisocyanate and polyol. Technologies of urethane were brought to the United States in 1953 by Jean-Pierre Abbat and Fritz Hartmann.

Polyurethanes can be manufactured with a large variety of textures and hardness's by varying the monomers used and by adding other substances.
They are used for the adhesives, paintings, elastomers ("rubber"), foams, fibres. Thus, these plastics with the vast applications are used in a great number of industries.
In the years 1970, the use of urethane for the wheels revolutionized the sports on casters (roller skate, board with casters).
Polyurethane material is characterised by outstanding characteristics, which make it interesting for a large range of applications:
excellent mechanical wear resistance
high rebound resilience, even in hard grades
good tear propagation resistance
low compression set
good stability against mineral oils, greases, gasoline and different solvents
Abrasion resistance:
The most known property of PU is its abrasion resistance. According to standard tests, the abrasion resistance of polyurethanes is about two and a half to five times higher than for many rubber raw materials and about three to four times higher than that of soft PVC. The differences in the field are frequently even greater, since the excellent damping and impact resilience of polyurethane does not come into play with the standard test methods.
Low temperature flexibility:
Polyurethane becomes increasingly harder with lower temperature, but in contrast to many other plastics, it does not become brittle. Thus the polyurethanes withstood the notched bar impact test even at -30°C without breaking. The long molecular chains (high molecular weights) of the raw materials give significantly better flexibility at low temperatures
Fire behaviour:
(The following information does not apply to electrically conductive, antistatic and flame-resistant types.) Like all organic materials, polyurethane is combustible. The toxicity of the combustion gases and smoke density are usually measured according to DIN 53436. Judged on the basis of this leading international standard, the potential release of hazardous substances (acute inhalation toxicity) at 800°c is no worse than that of natural products such as wood, wool or leather.
